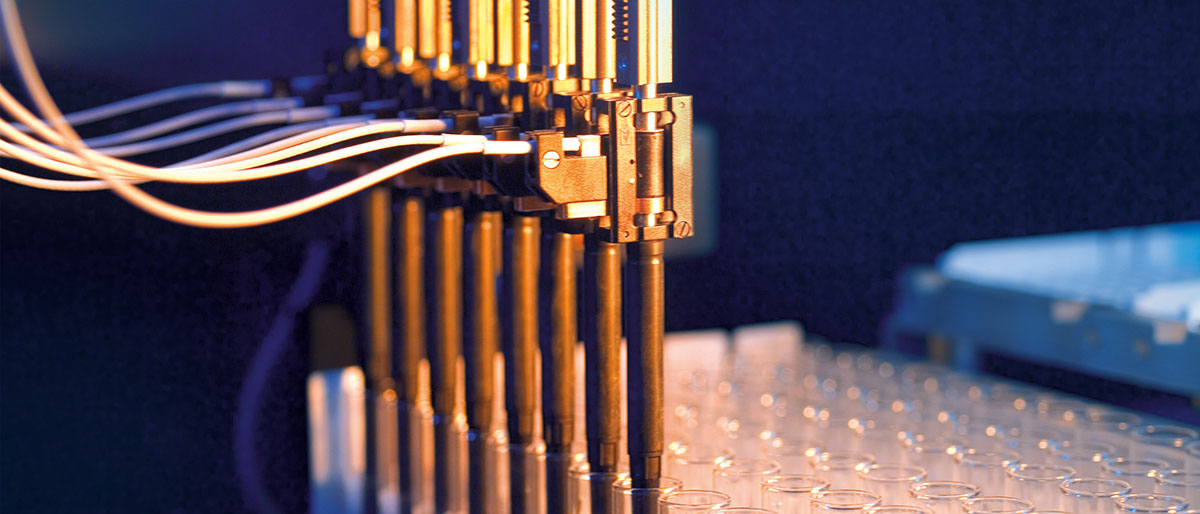
Reformat entire compound libraries to suit your requirements

Outsourcing compound library storage
in one of our manual or automated stores

Maintain integrity and purity of compounds
with our Quality Control services

Cost efficiently outsource active
compound repository operations to Specs

Design targeted compound libraries
or analyze chemistry data using our cheminformatics services
